CAT #: 12070101
T-Cell Receptor Gamma Gene Rearrangement Assay 2.0
Assay Use
This Research Use Only assay identifies T-cell receptor gamma (TCRG) chain gene rearrangements and is useful for the identification of clonal T-cell populations and evaluation of new research and methods in malignancy studies.
Product Details
-
Summary and Explanation of the Test
BACKGROUND
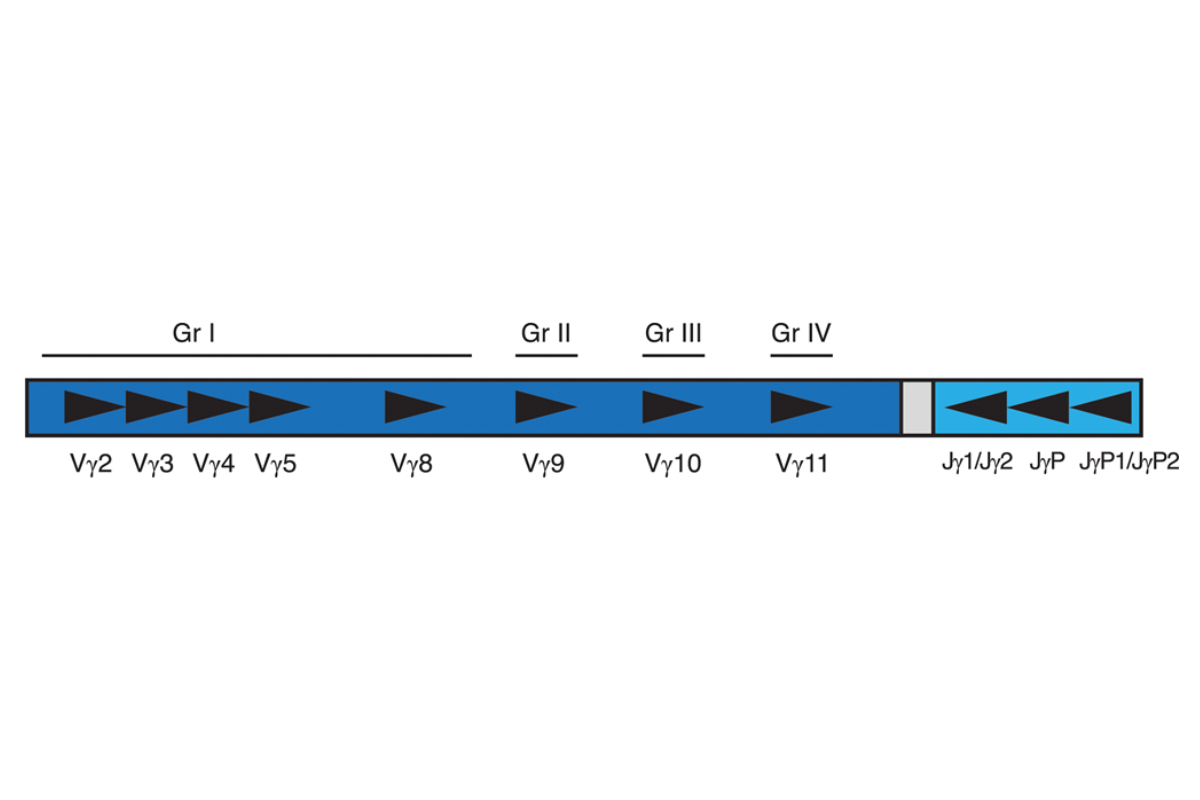
Invivoscribe’s T-Cell Receptor Gamma Gene Rearrangement Assay 2.0 represents an improved approach to PCR-based clonality testing of T-cell lymphoproliferative disorders. This assay was optimized using a single amplification reaction with a single fluorescent dye for detection. Amplified products generated targeting the TCR gamma gene locus all fall within a single size range to facilitate interpretation.The human TCR gamma gene locus on chromosome seven (7q14) includes 14 V genes (6 of these V genes are functional; 3 open reading frames and 5 pseudogenes) belonging to four subgroups (Group I, II, III and IV), 5 J segments and 2 C genes spread over 200 kilobases. The diversity of this locus has complicated PCR-based testing. This multiplex PCR assay represents an improvement over existing assays as it can detect the vast majority of TCR gamma gene rearrangements with a single multiplex master mix. Importantly, this assay includes primers for all known groups of TCR gamma variable region genes and joining region genes involved in rearrangements in T-cell lymphomas. In addition, all labeled primers are conjugated with the 6FAM fluorophore.
SUMMARY
Our single multiplex master mix targets all conserved regions within the variable (V) and the joining (J) region genes that are described in lymphoid malignancies. This is critical for more comprehensive analysis of samples, as some T-cell lymphoproliferative disorders involve V and J segments that would not be identified with a single Vγ (1-8) and Jγ1/2 primer set. Amplification with all primers in a single tube has several additional important advantages over existing methods. The polyclonal background that results from the combination of all primers in a single tube produces a more robust and easily interpreted signal with capillary electrophoresis, which aids in the interpretation of small peaks. The average size of the TCRG gene rearrangement PCR product is 190 bp, with a normal distribution of product sizes between 159 and 207 bp. This protocol leads to improved PCR product formation from paraffin-embedded samples compared to other protocols that yield products of 260 bp or longer. Positive and negative DNA controls, as well as a Specimen Control Size Ladder master mix are included. Clonality is indicated if a dominant amplicon is detected.This test kit includes the TCRG – 6FAM master mix that targets framework regions within the variable region and the joining region of the TCR gamma chain locus. The second master mix, the Specimen Control Size Ladder, targets multiple genes and generates a series of amplicons of 100, 200, 300, 400, and 600 bp that can be used to ensure that the quality of input DNA is adequate to yield a valid result.
-
Specimen Requirements
This assay tests extracted and purified genomic DNA (gDNA). Common sources of gDNA include:
- 5 cc of peripheral blood, bone marrow biopsy or bone marrow aspirate anti-coagulated with heparin or EDTA; OR
- Formalin-fixed paraffin embedded tissue or slides.
Related Products
Disclaimer
This assay was developed by Invivoscribe. The performance of this assay was reviewed and validated by the EuroClonality/BIOMED-2 Group.
Legal Notice
Warranty and Liability
Invivoscribe, Inc. (Invivoscribe®) is committed to providing the highest quality products. Invivoscribe® warrants that the products meet or exceed the performance standards described in the Instructions For Use, as to products with such an insert. If a product is covered by product specifications and does not perform as specified, our policy is to replace the product or credit the full purchase price. No other warranties of any kind, expressed or implied, are provided by Invivoscribe®. Invivoscribe® liability shall not exceed the purchase price of the product. Invivoscribe® shall have no liability for direct, indirect, consequential or incidental damages arising from the use, results of use, or inability to use its products; product efficacy under purchaser controlled conditions in purchaser’s laboratory must be established and continually monitored through purchaser defined and controlled processes including but not limited to testing of positive, negative, and blank controls every time a sample is tested. Ordering, acceptance, and use of product constitutes purchaser acceptance of sole responsibility for assuring product efficacy and purchaser agreement to the limitation of liability set forth in this paragraph.
This product is for Research Use Only; not for use in diagnostic procedures.
This product is covered by one or more of the following: European Patent Number 1549764, European Patent Number 2418287, European Patent Number 2460889, Japanese Patent Number 4708029, United States Patent 8859748, United States Patent 10280462, and related pending and future applications. All of these patents and applications are licensed exclusively to Invivoscribe®. Additional patents licensed to Invivoscribe® covering some of these products apply elsewhere. Many of these products require nucleic acid amplification methods such as Polymerase Chain Reaction (PCR). No license under these patents to use amplification processes or enzymes is conveyed expressly or by implication to the purchaser by the purchase of this product.
©2024 Invivoscribe, Inc. All rights reserved. The trademarks mentioned herein are the property of Invivoscribe, Inc. and/or its affiliates, or (as to the trademarks of others used herein) their respective owners.

 Mexico and Canada
Mexico and Canada  Outside North America
Outside North America  US
US