CAT #: 91010081
IdentiClone® IGH Gene Clonality Assay MegaKit - ABI Fluorescence Detection
Intended Use
The IdentiClone® IGH Gene Clonality Assay is an in vitro diagnostic product intended for PCR-based detection of clonal immunoglobulin heavy chain gene rearrangements in patients with suspect lymphoproliferations. Specifically, the IGH Gene Clonality Assay can be used to:
- Identify clonality in atypical lymphoproliferative disorders
- Support a differential diagnosis between reactive lesions and hematologic malignancies3
- Assign presumptive lineage in mature monoclonal lymphoproliferative disorders
- Identify tumor-specific markers (IGH gene rearrangements) for post-treatment monitoring
- Monitor and evaluate disease recurrence
Product Details
-
Summary and Explanation of the Test
Rearrangements of the antigen receptor genes occur during ontogeny in B and T lymphocytes. These gene rearrangements generate products that are unique in length and sequence for each cell. Therefore, polymerase chain reaction (PCR) assays can be used to identify lymphocyte populations derived from a single cell by detecting the unique V-J gene rearrangements present within these antigen receptor loci.1 This PCR assay employs multiple consensus DNA primers that target conserved genetic regions within the immunoglobulin heavy chain gene. This test is used to detect the vast majority of clonal B-cell malignancies from DNA. Test products can be analyzed using a variety of detection formats, including gel and capillary electrophoresis.
Invivoscribe’s IdentiClone assays represent a simple approach to PCR-based clonality testing. These standardized assays were carefully optimized testing positive and negative control samples using multiplex master mixes. Assay development was followed by extensive validation including the testing of more than 400 clinical samples using Revised European/American Lymphoma (REAL) classification. Testing was performed at more than thirty prominent independent testing centers throughout Europe in a collaborative study known as the BIOMED-2 Concerted Action.2
The ABI detection based assays cannot reliably detect clonal populations comprising less than 1% of the total lymphocyte cell population. Always interpret the results of molecular clonality tests in the context of clinical, histological and immunophenotypic data.
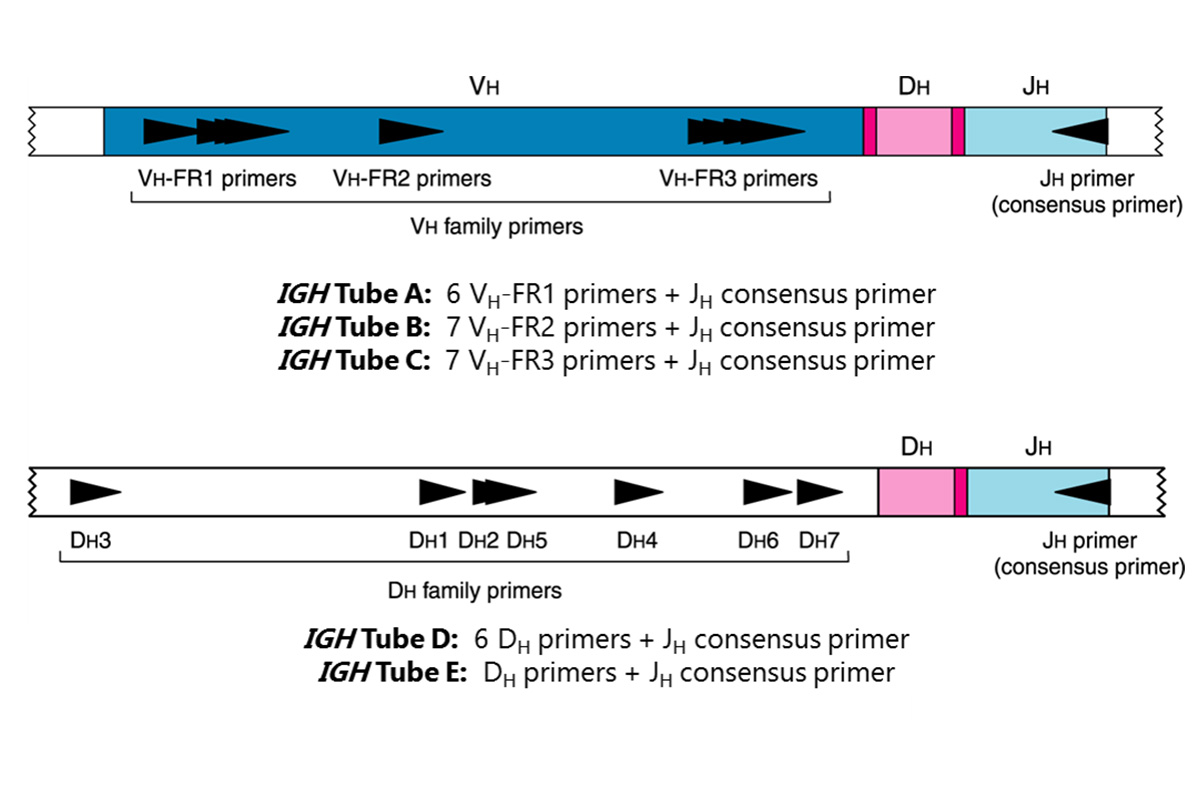
This test kit includes six (6) master mixes. The IGH Tube A, B, and C master mixes target the framework 1, 2, and 3 regions (respectively) within the variable region, and the joining region of the immunoglobulin heavy chain locus. The IGH Tubes D and E master mixes target the diversity and joining regions. Lastly, the Specimen Control Size Ladder master mix, targets multiple genes and generates a series of amplicons of approximately 100, 200, 300, 400, and 600 base pairs (bp) to ensure that the quality and quantity of input DNA is adequate to yield a valid result. A single thermal cycler program and similar detection methodologies are used with all Invivoscribe Gene Clonality Assays. This improves consistency and facilitates cross training on a broad range of different assays.
-
Principles of the Procedure
Polymerase Chain Reaction (PCR)
PCR assays are routinely used for the identification of clonal B-cell populations. These tests amplify the DNA between primers that target the conserved framework (FR) of the variable (V) regions and the conserved joining (J) regions (IGH Tubes A, B, and C), as well as the diversity (D) and joining regions (IGH Tubes D and E. These conserved regions lie on either side of an area within the V-J region where programmed genetic rearrangements occur during maturation of all B and T lymphocytes. The antigen receptor genes that undergo rearrangement are the immunoglobulin heavy chain and light chains in B-cells, and the T cell receptor genes in T-cells. Each B- and T-cell has a single productive V-J rearrangement that is unique in both length and sequence. Therefore, when DNA from a normal or polyclonal population is amplified using DNA primers that flank the V-J region, a bell-shaped curve (Gaussian distribution) of amplicon products within an expected size range is generated. This Gaussian distribution reflects the heterogeneous population of V-J rearrangements. (In certain cases, where lymphocyte DNA is not present, no product is observed.) DNA from samples containing a clonal population yield one or two prominent amplified products (amplicons) within a diminished polyclonal background.
Since the antigen receptor genes are polymorphic (consisting of a heterogeneous population of related DNA sequences), it is difficult to employ a single set of DNA primer sequences to target all of the conserved flanking regions around the V-J rearrangement. N-region diversity and somatic mutation further scramble the DNA sequences in these regions. Therefore multiplex master mixes, which target several FR regions, are required to identify the majority of clonal rearrangements. As indicated, clonal rearrangements are identified as prominent, single-sized products within the background of different-sized amplicon products that form a Gaussian distribution around a statistically favored, average-sized rearrangement. Note that the primers that amplify the different FR regions, which are located at three distinct sections along the heavy chain gene, produce a correspondingly different size-range of V-J products.
Differential Fluorescence Detection
Differential fluorescence detection is commonly used to resolve the different-sized amplicon products using a capillary electrophoresis instrument. Primers can be conjugated with several different fluorescent dyes (fluorophors) so that they can produce different emission spectra upon excitation by a laser in the capillary electrophoresis instrument. In this manner, different fluorescent dyes can correspond to different targeted regions. This detection system results in unsurpassed sensitivity, single nucleotide resolution, differential product detection, and relative quantification. In addition, the use of agarose and polyacrylamide gels, as well as the use of carcinogens such as ethidium bromide, can virtually be eliminated. Further, differential detection allows accurate, reproducible and objective interpretation of primer-specific products and automatic archiving of data. Inter-assay and intra-assay reproducibility in size determination using capillary electrophoresis is approximately 1 to 2 nucleotides. This reproducibility and sensitivity coupled with the automatic archiving of specimen data allows for the monitoring, tracking, and comparison of data from individual patients over time.
-
Specimen Requirements
This assay tests genomic DNA (gDNA) from the following sources:
- 5 cc of peripheral blood, bone marrow biopsy, or bone marrow aspirate anti-coagulated with heparin or EDTA (stored at 2°C to 8°C and shipped at ambient temperature)
- Minimum 5 mm cube of tissue (stored and shipped frozen; or stored and shipped in RPMI 1640 at ambient temperature or on ice)
- 3 µg of gDNA (stored at 2°C to 8°C and shipped at ambient temperature)
- Formalin-fixed paraffin embedded tissue or slides (stored and shipped at ambient temperature)
References
1. Miller, JE et al. (1999). Molecular Diagnostics. 4(2):101-117.
2. Van Dongen, JJM et al. (2003). Leukemia. 17(12):2257-2317.
3. van Krieken, JHJM et al. (2007) Leukemia. 21(3):201-206.
Disclaimer
This assay is based on the EuroClonality/BIOMED-2 Concerted Action BMH4-CT98-3936.
Legal Notice
Warranty and Liability
Invivoscribe, Inc. (Invivoscribe®) is committed to providing the highest quality products. Invivoscribe® warrants that the products meet or exceed the performance standards described in the Instructions For Use, as to products with such an insert. If a product is covered by product specifications and does not perform as specified, our policy is to replace the product or credit the full purchase price. No other warranties of any kind, expressed or implied, are provided by Invivoscribe®. Invivoscribe® liability shall not exceed the purchase price of the product. Invivoscribe® shall have no liability for direct, indirect, consequential or incidental damages arising from the use, results of use, or inability to use its products; product efficacy under purchaser controlled conditions in purchaser’s laboratory must be established and continually monitored through purchaser defined and controlled processes including but not limited to testing of positive, negative, and blank controls every time a sample is tested. Ordering, acceptance, and use of product constitutes purchaser acceptance of sole responsibility for assuring product efficacy and purchaser agreement to the limitation of liability set forth in this paragraph.
This product is an in vitro diagnostic product is not available for sale or use within North America.
This product is covered by one or more of the following: European Patent Number 1549764, European Patent Number 2418287, European Patent Number 2460889, Japanese Patent Number 4708029, United States Patent 8859748, United States Patent 10280462, and related pending and future applications. All of these patents and applications are licensed exclusively to Invivoscribe®. Additional patents licensed to Invivoscribe ®covering some of these products apply elsewhere. Many of these products require nucleic acid amplification methods such as Polymerase Chain Reaction (PCR). No license under these patents to use amplification processes or enzymes is conveyed expressly or by implication to the purchaser by the purchase of this product.
IdentiClone® is a registered trademark of Invivoscribe®.
©2024 Invivoscribe, Inc. All rights reserved. The trademarks mentioned herein are the property of Invivoscribe, Inc. and/or its affiliates, or (as to the trademarks of others used herein) their respective owners.

 Outside North America
Outside North America